YTY INDUSTRY SDN BHD
Integra Tower, #19-01,
Intermark, 348 Jalan Tun Razak,
50400, Kuala Lumpur,
Malaysia
Phone No : 603 2177 0000
Fax No : 603 2177 0002
Email : laura.chong@ytygroup.com.my
Phone No : 603 2177 0000
Fax No : 603 2177 0002
Email : laura.chong@ytygroup.com.my

Click on image
Click image to enlarge
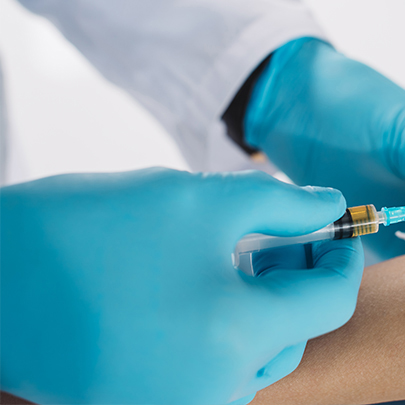
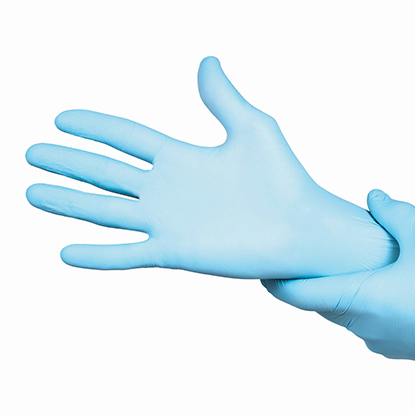
Freedom IV, the industry�s lowest-weight Accelerator-Free Nitrile Examination glove that is compliant with both US ASTM and European EN regulatory requirements. As such, Freedom IV will be able to offer a �Low Dermatitis Potential� labelling claim, as supported by clinical testing. Given the unique formulation used to manufacture it, Freedom IV is also sulphur-free.
Freedom IV represents a new paradigm in synthetic hand-protection solutions. Made with a unique formulation, it is Accelerator-Free, meaning it is made completely without any allergy-causing chemical accelerators. At 3.2g, it is a uniquely low-weight Accelerator-Free Nitrile Examination glove in the market, and is competitively priced so as to be perfectly positioned for Acute and Alternate care environments alike.
Freedom IV represents a new paradigm in synthetic hand-protection solutions. Made with a unique formulation, it is Accelerator-Free, meaning it is made completely without any allergy-causing chemical accelerators. At 3.2g, it is a uniquely low-weight Accelerator-Free Nitrile Examination glove in the market, and is competitively priced so as to be perfectly positioned for Acute and Alternate care environments alike.
Leave Your Message
Freedom IVâs unique formulation is protected via intellectual property rights and a proprietary process of manufacture developed by YTY. Freedom IV is supported with a valid US FDA 510k, which means orders can be placed as soon as you are ready to take it to market with your end-customers.